New EsophyX Z+ device supports TIF 2.0 procedure with feature set of EsophyX Z device and benefits of adult sized endoscopes.
REDMOND, Wash. – EsophyX® Z+ device offers expanded endoscope compatibility and joins EndoGastric Solutions’® portfolio of specialized technologies used to perform the TIF 2.0 procedure to reconstruct the gastroesophageal valve and restore its function as a reflux barrier.
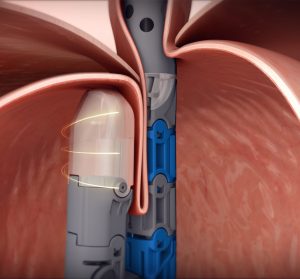
EsophyX Z+ model device used in TIF 2.0 procedure rebuilding gastroesophageal valve with 270º wrap and 3cm length
Using a transoral approach, the EsophyX Z+ device enables physicians to rebuild the gastroesophageal valve with a partial fundoplication technique that secures the tissue with dual deployment of SerosaFuse® Implantable Fasteners.
Michael Murray, M.D., FACS, Northern Nevada Medical Center and Kenneth Chang, M.D., UC Irvine Health were the first to perform the TIF 2.0 procedure using the new EsophyX Z+ device. Both clinicians are well-versed in the TIF 2.0 procedure and have been treating patients with severe gastroesophageal reflux disease (GERD) symptoms for nearly a decade.
“GERD is a chronic condition and proton pump inhibitors do not control symptoms in about one-third of patients,” says surgeon Michael Murray, M.D., Northern Nevada Medical Center. “The design of the new EsophyX Z+ device makes performing TIF 2.0 procedures technically easy and produces a consistent and effective fundoplication thus providing an effective alternative to prescription medications and traditional surgical procedures.”
“The robust collection of clinical data and recent positive policy coverage announcements support the TIF 2.0 procedure as an established treatment option for patients who wish to eliminate or discontinue PPI use and avoid surgery for acid reflux. This newest design iteration allows for maximal visibility using a standard endoscope combined with almost effortless and confident deployment of fasteners. GI endoscopists and surgeons should consider the TIF procedure in patients seeking a less invasive, proven GERD treatment solution,” adds Gastroenterologist Kenneth Chang, M.D., UC Irvine Health.
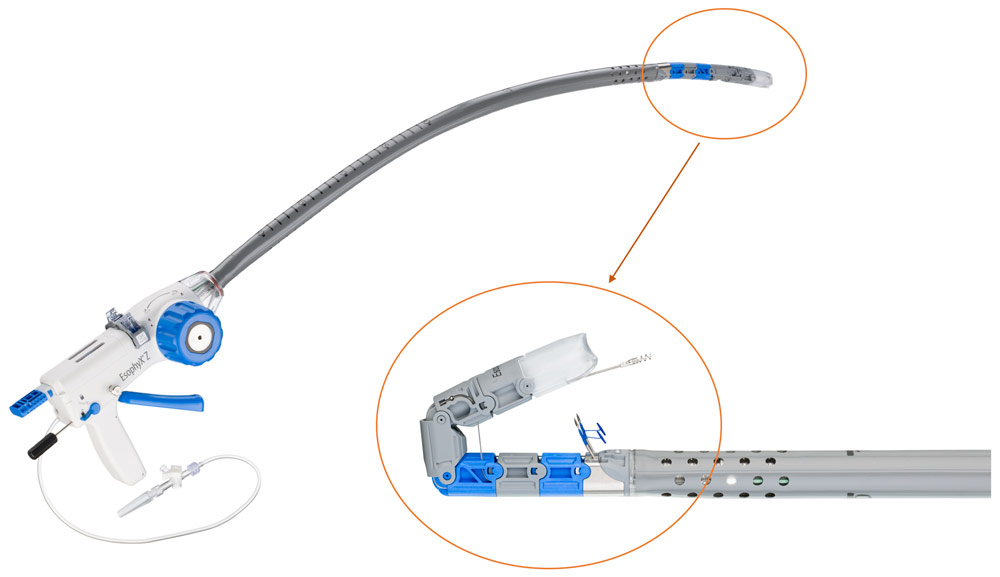
EsophyX Z+ model device with close up of tissue mold showing dual fastener deployment
The original EsophyX device was cleared by the FDA in 2007. EGS launched the third generation EsophyX Z model platform in 2015. The evolving EsophyX Z+ technology now enables surgeons and gastroenterologists to use a wider selection of standard endoscopes to treat the underlying anatomical cause of GERD.
“We are excited to launch the EsophyX Z+ device as we believe it will further enhance the physician’s technical experience,” says Skip Baldino, President and CEO of EndoGastric Solutions. “At EndoGastric Solutions, we are committed to addressing the unmet needs in gastrointestinal diseases and offering physicians highly effective options that fill the significant treatment gap between medication and more invasive surgery.”
NP02450-26A/-01G
